Regulation And Validation
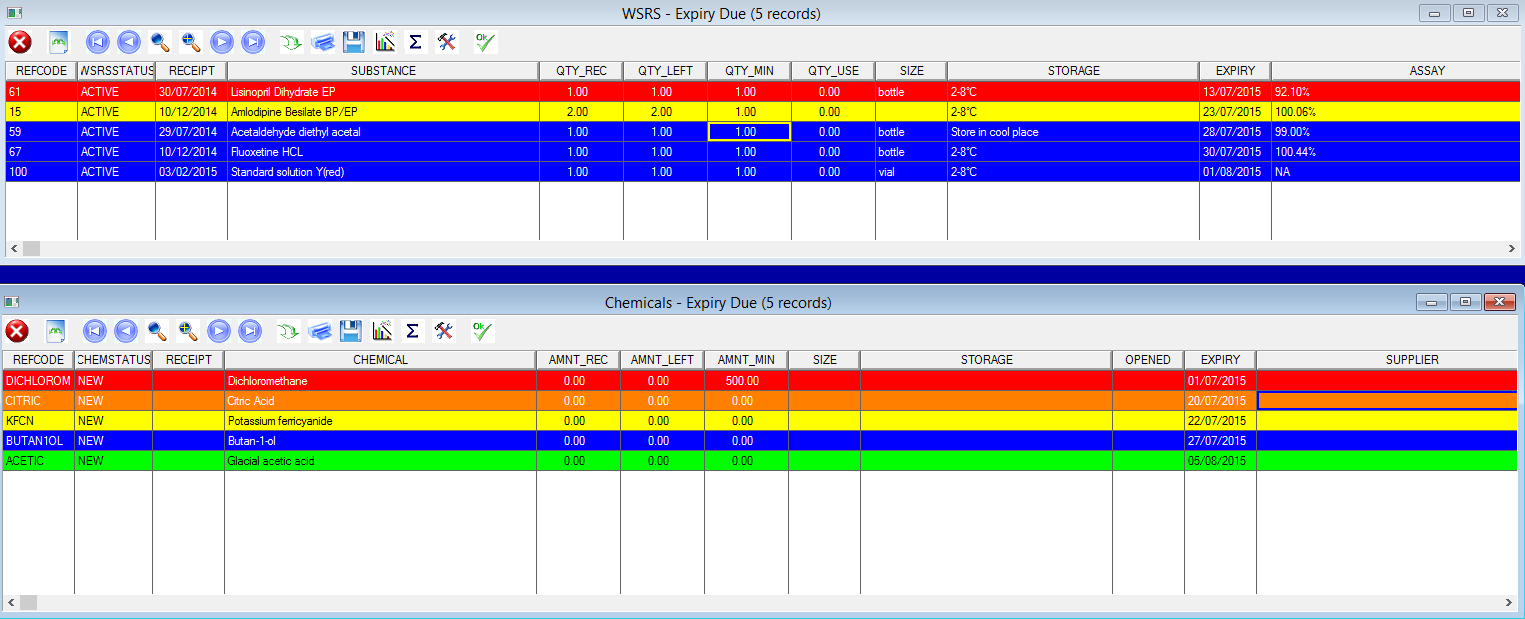
Laboratories in this industry are inundated with testing requests, repeats, screening and validation testing. This understandably creates a large quantity of data and requires a suitable platform with which to manage this. Laboratories in this industry also face immense pressure to be regulatory compliant and experience an increased pressure to improve efficiency and productivity.
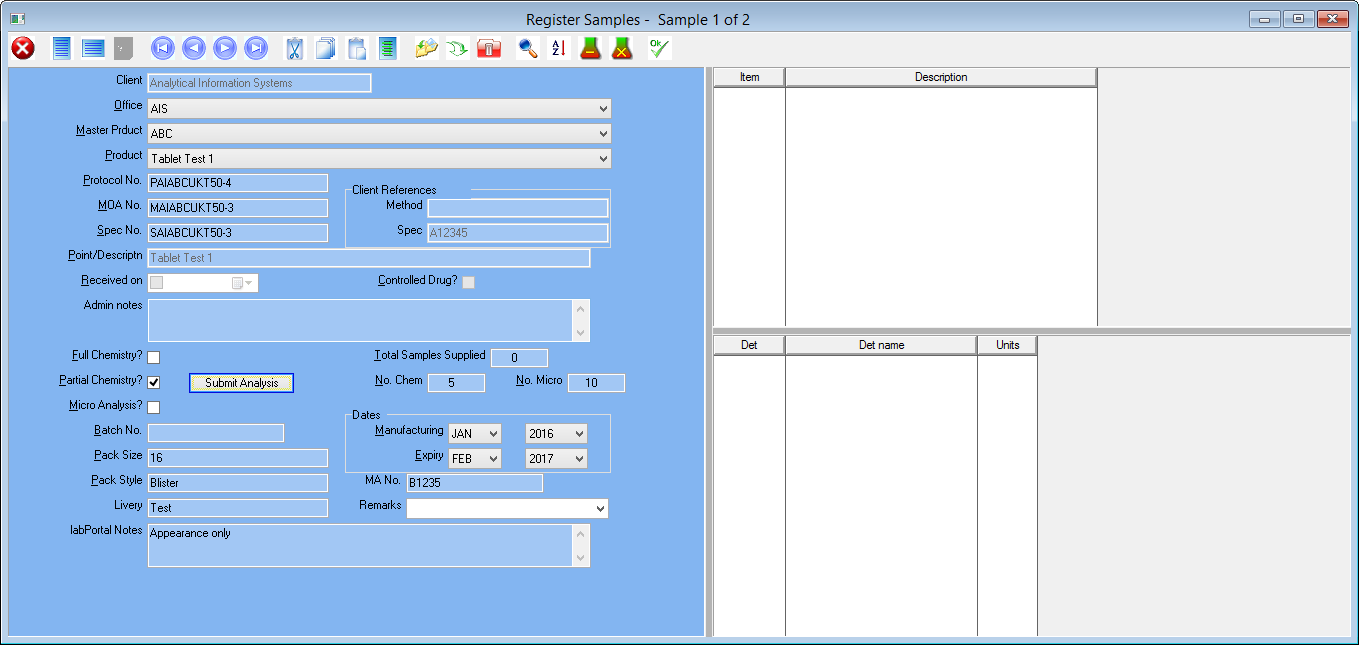
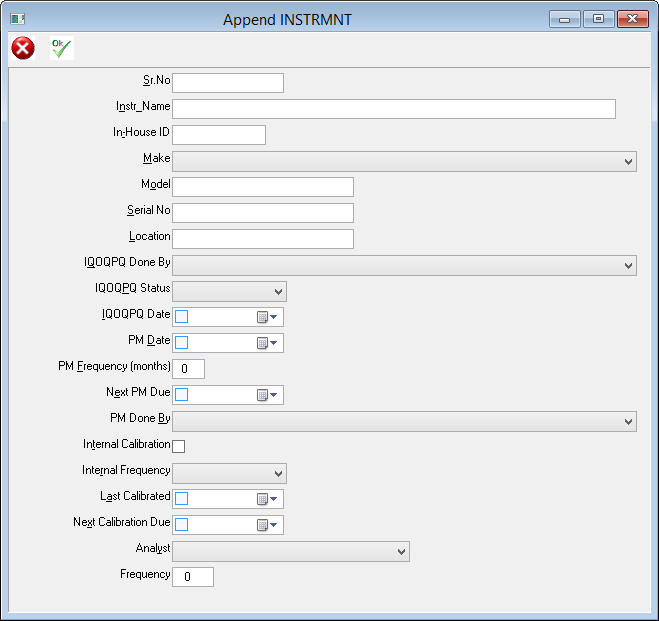
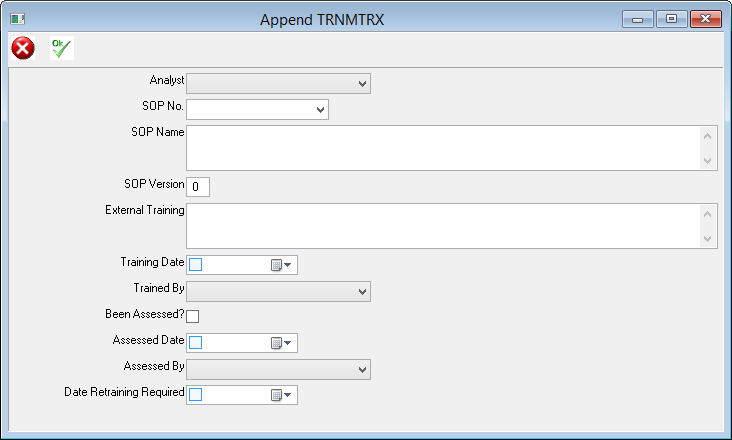
Our AIS LIMS solutions are necessary for pharmaceutical laboratories to address these issues. AIS are able to configure a suitable package to suit you, your business needs and requirements. We have a team of knowledgeable, experienced staff who are familiar with regulatory guidelines such as FDA’s 21 CFR part 11 and the importance of audit trails and sample traceability. AIS LIMS will integrate with your various instruments and allow for the automation of analysis and data collection. In doing so the opportunity of obtaining transcription errors is reduced, sample turnaround is improved and lab efficiency increases.
For more information about LIMS solutions from AIS Ltd call +44 (0) 1531 828930 or email marketing@ais-lims.com.